Ok. Now that that's out of the way. Onwards to Mole Land!
MOLES: THE AMOUNT OF SUBSTANCE
In five minutes you will not be confused about Moles ever again! Thank goodness! Thank you "Chemguy" for making the Mole Madness end!
WIKI: The amount of substance, n, of a sample or system is a physical quantity which is proportional to the number of elementary entities present.[1] "Elementary entities" may be atoms, molecules, ions, electrons, or particles, the choice of which is dependent upon context and must be stated. Amount of substance is sometimes referred to as chemical amount or, incorrectly, as number of moles.[2] Amount of substance is a quantity that measures the size of an ensemble of entities. It appears in thermodynamic relations such as the ideal gas law, and in stoichiometric relations between reacting molecules as in the law of multiple proportions. The SI unit for amount of substance is the mole (symbol: mol), which is defined as the amount of substance that has an equal number of elementary entities as there are atoms in 12 g of carbon-12.[3] That number is equivalent to the Avogadro constant, NA, which has a value[4] of 6.02214179(30)×1023 mol−1. The only other unit of amount of substance in current use is the pound mole (symbol: lb-mol.), which is sometimes used in chemical engineering in the United States.[5][6]
- 1 lb-mol. ≡ 453.592 37 mol (this relation is exact, from the definition of the international avoirdupois pound).
The Avogadro constant (symbols: L, NA), also called Avogadro's number, is the number of "elementary entities" (usually atoms or molecules) in one mole, that is (from the definition of the mole), the number of atoms in exactly 12 grams of carbon-12.[1][2] The 2006 CODATA6.02214179(30)×1023 entities per mole.[3] recommended value is
LENGTH
The image above is a micrograph of a (nanowire curled into a loop in front of a strand of human hair. The nanowires can be as slender as 50 nanometers in width, about one-thousandth the width of a hair. Credit: Limin Tong/Harvard University
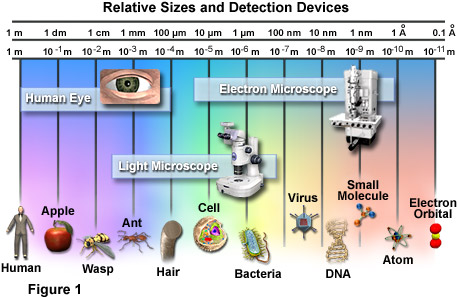
WIKI: A nanometer, symbol nm, is a unit of length in the metric system, equal to one billionth of a metre (i.e., 10-9 m or one millionth of a millimetre). It is one of the more often used units for very small lengths, and equals ten Ångström, an internationally recognized non-SI unit of length. It is often associated with the field of nanotechnology. It is also the most common unit used to describe the manufacturing technology used in the semiconductor industry. It is the most common unit to describe the wavelength of light, with visible light falling in the region of 400–700 nm. The data in compact discs is stored as indentations (known as pits) that are approximately 100. Formerly, millimicron (symbol mµ) was used for the nanometre. The symbol µµ has also been used [1][2][3].
FORCE
The newton (symbol: N) is the SI derived unit of force, named after Isaac Newton in recognition of his work on classical mechanics.The newton is the unit of force derived in the SI system; it is equal to the amount of force required to give a mass of one kilogram an acceleration of one meter per second squared. Algebraically: Examples:
- 1 N is the force of Earth's gravity on an object with a mass of about 102 g (1⁄9.8 kg) (such as a small apple).
- On Earth's surface, a mass of 1 kg exerts a force of approximately 9.80665 N [down] (or 1 kgf). The approximation of 1 kg corresponding to 10 N is sometimes used as a rule of thumb in everyday life and in engineering.
- The decanewton (daN) = 10 N is increasingly used when specifying load bearing capacity of items such as ropes and anti-vibration mounts because it is approximately equivalent to the more familiar non-SI unit of force, the kgf.
- The force of Earth's gravity on a human being with a mass of 70 kg is approximately 687 N.
- The dot product of force and distance is mechanical work. Thus, in SI units, a force of 1 N exerted over a distance of 1 m is 1 N·m of work. The Work-Energy Theorem states that the work done on a body is equal to the change in energy of the body. 1 N·m = 1 J (joule), the SI unit of energy.
- It is common to see forces expressed in kilonewtons or kN, where 1 kN = 1 000 N.
WIKI: The joule is the derived unit of energy in the International System of Units. It is defined as:
One joule is the amount of energy required to perform the following actions:
- The work done by a force of one newton traveling through a distance of one meter;
- The work required to move an electric charge of one coulomb through an electrical potential difference of one volt; or one coulomb volt, with the symbol C·V;
- The work done to produce power of one watt continuously for one second; or one watt second (compare kilowatt hour), with the symbol W·s. Thus a kilowatt hour is 3,600,000 joules or 3.6 megajoules;
- The kinetic energy of a 2 kg mass moving at a velocity of 1 m/s. The energy is linear in the mass but quadratic in the velocity, being given by E = ½mv²;
1 joule is approximately equal to:
- 6.2415 ×1018 eV (electronvolts)
- 0.2390 cal (calorie) (small calories, lower case c)
- 2.3901 ×10−4 kilocalorie, Calories (food energy, upper case C)
- 9.4782 ×10−4 BTU (British thermal unit)
- 0.7376 ft·lbf (foot-pound force)
- 23.7 ft·pdl (foot poundals)
- 2.7778 ×10−7 kilowatt hour
- 2.7778 ×10−4 watt hour
- 9.8692 ×10−3 litre-atmosphere
Units defined in terms of the joule include:
- 1 thermochemical calorie = 4.184 J
- 1 International Table calorie = 4.1868 J
- 1 watt hour = 3600 J
- 1 kilowatt hour = 3.6 ×106 J (or 3.6 MJ)
- 1 ton TNT exploding = 4.184 GJ
Useful to remember: 1 joule = 1 newton meter = 1 watt second
Practical examples. One joule in everyday life is approximately:- the energy required to lift a small apple one meter straight up.
- the energy released when that same apple falls one meter to the ground.
- the energy released as heat by a quiet person, every hundredth of a second.
- the energy required to heat one gram of dry, cool air by 1 degree Celsius.
- one hundredth of the energy a person can receive by drinking a drop of beer.
- the kinetic energy of an adult human moving a distance of about a handspan every second.
WIKI: The pascal (symbol: Pa) is the SI derived unit of pressure, stress, Young's modulus and tensile strength. It is a measure of perpendicular force per unit area i.e. equivalent to one newton per square meter or one joule per cubic metre. In everyday life, the pascal is perhaps best known from meteorological barometric pressure reports, where it occurs in the form of hectopascals (1 hPa = 100 Pa).[1]. In other contexts, the kilopascal is more commonly used, for example on bicycle tire labels.[2] One hectopascal corresponds to about 0.1% and one kilopascal to about 1% of atmospheric pressure (near sea level): one hectopascal is thus equivalent to a millibar; one atmosphere is equal to 1013.25 hPa. The pascal (Pa) or kilopascal (kPa) as a unit of pressure measurement is widely used throughout the world and largely replaces the pounds per square inch (psi) unit except in some countries still using the Imperial measurement system. Vehicle owners' guides now specify tire inflation in kilopascals.
1 pascal (Pa) ≡ 1 N/m2 ≡ 1 J/m3 ≡ 1 kg/(m·s2)
And here's an online converter you can play around with: http://www.imperialtometric.com/conversion_en.htm
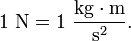

0 komentar:
Posting Komentar