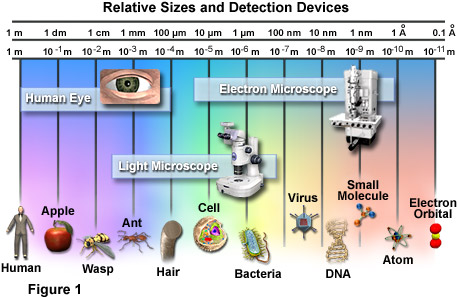
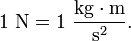Here is a more up to date Biology documentary (2003) also from Oregon Public Broadcasting.
It's available in streaming format only,
so you first you need to go to www.learner.org and sign up for free. Then make sure you enable pop ups and click just on the links below.This documentary also has its own web site with an Online Textbook, Case Studies, expert Interview Transcripts, Image & Animation Archive, and a Glossary!
http://www.learner.org/courses/biology/index.htmlRediscovering Biology: Molecular to Global Perspectives(2003) "Great advances have been made in the field of biology in recent decades that will continue to have a major impact on our lives.
Rediscovering Biology: Molecular to Global Perspectives explains these developments for teachers of high school biology to update their content knowledge and understanding. The multimedia course materials—video, online text, interactive Web activities, and course guide—will help new and veteran biology teachers become familiar with current research methods and tools that will lead to new discoveries in the coming decades. Thirteen half-hour video programs feature interviews with expert scientists involved in groundbreaking research, such as Eric Lander of the MIT Genomics Center and Rita Colwell, director of the National Science Foundation. Detailed animations provide a micro-level view of biological processes and techniques such as mass spectrometry and microarray analysis. Supporting and expanding the video content, the course guide and interactive Web site provide learning activities, additional information, a detailed glossary, annotated animations, and case studies that invite teachers to run their own mini research projects. An extensive online text, downloadable for printing, covers the content participants need to know for the 13 units. Produced by Oregon Public Broadcasting."
Session 1. GenomicsHaving determined the complete DNA nucleotide sequence of humans and several other organisms, today’s research has shifted to identifying genes and determining their functions. This session reviews the techniques used in BLAST searches, microarray experiments, and other genomics tools.
Session 2. Proteins and ProteomicsResearchers know it is the proteins made by a cell that determine what that cell does. This session explores the varying complements of proteins and their effects, structures, and interactions within the mechanism of cell function, and introduces the larger picture of proteomics and systems biology.
Session 3. Evolution and PhylogeneticsThe ability to compare DNA sequences from different organisms is refining our perspective on evolution. This session illustrates how molecular techniques are now combined with fossil evidence to explore relationships in organisms from whales to anthrax.
Session 4. Microbial DiversityMicrobial diversity far surpasses all other diversity on the planet. This session examines recent studies of microbes including extremophiles, the comparisons of Bacteria and Archaea, and the formation and life cycle of biofilms.
Session 5. Emerging Infectious DiseasesNew diseases arise and old diseases, such as malaria and influenza, are returning with renewed vigor. This session studies the complex causes and far-reaching impacts of emerging infectious diseases around the globe.
Session 6. HIV and AIDSStudying individuals with natural resistance to HIV has led to insights into the infection process and may produce new treatments or a vaccine. This session explores recent developments in the study of HIV and AIDS, the future global impact of the current infection levels, and the ethical issues surrounding current research and treatments.
Session 7. Genetics of DevelopmentOrganisms as different as flies, fish, and humans share a set of genes, known as a genetic toolkit, which guides development. This session presents new perspectives on the remarkable similarity in these molecules and processes and the ethical questions involved in this research.
Session 8. Cell Biology and CancerCancers result when genes required for normal cell function are mutated and the resulting cells undergo other changes ultimately leading to uncontrolled division. This session reveals new information on normal cell function, proto-oncogenes and tumor suppressor genes and their role in the cell cycle, and current research in drug design for specific cancers.
Session 9. Human EvolutionHomo sapiens is now the only living representative of what was once a multi-branched bush of hominid species. This session examines mitochondrial Eve and other fossil clues that increasingly point to Africa as the point of origin of our species. How did humans replace their hominid cousins, including Neanderthal, leaving the chimpanzee as our closest living relative?
Session 10. NeurobiologyNeurons’ electrical activity results in the release of neurotransmitters that account for everything from survival to addiction to learning and memory. This session explains how neurons communicate to achieve all these functions.
Session 11. Biology of Sex and GenderSeveral genes help determine what makes a human embryo develop female or male sexual anatomies. This session examines recent findings which have challenged previous beliefs about the roles of anatomy, environment, and genetics in the determination of gender, and the evolution of sexual determination.
Session 12. BiodiversityWith current extinction rates exceeding those of previous mass extinctions, many biodiversity studies focus on efforts to count the Earth’s species before they are lost. This session explores current field experiments studying complex ecosystems and how environmental and biodiversity changes might affect their functions.
Session 13. Genetically Modified OrganismsWhile genetic modification of organisms has occurred for millennia, we now have the tools to insert specific genes from one organism into cells of unrelated species. This session illustrates the processes used and how such genetically transformed organisms are increasingly common in agriculture, industry, and medicine, and introduces the ethical considerations of GMO research.
 Iceland has intrigued human population geneticists for some time and Icelanders are one of the most studied human populations because of their relatively small population and relatively large degree of isolation. These two features combine to give genetic drift more power than in most populations. A paper in PLoS Genetics this month spells out exactly how important genetic drift has been. There's a report on the paper here.
Iceland has intrigued human population geneticists for some time and Icelanders are one of the most studied human populations because of their relatively small population and relatively large degree of isolation. These two features combine to give genetic drift more power than in most populations. A paper in PLoS Genetics this month spells out exactly how important genetic drift has been. There's a report on the paper here.